Answer 1 of 2. No hydrogen bonding obviously because for that the hydrogen atoms need to be bonded to one of N O or F No dipole-dipole forces since H-H is totally non polar.
The Shape Of The Molecules Of Hydrogen Sulphide And Water Are Similar Yet Their Boiling Point Differ Why Quora
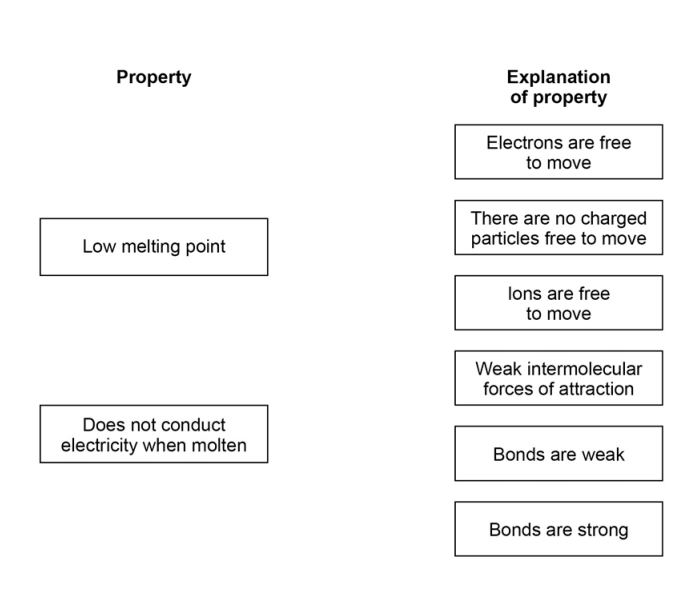
Low melting and boiling points - this is because little energy is needed to break the weak intermolecular forces.
. H2S is not a strong acid. Because of this comparatively weak intermolecular forces exist for H2S and the melting and boiling points are much lower than they are in water. Hydrogen sulfide and water boil at -607 oC and 1000 oC respectively.
Because it has very weak intermolecular forces. For H2S there is only 1 H2S does not disassociate lower boiling point elevation. And as chemists as physical scientists we should interrogate actual data and list the normal boiling points so.
2 SO2 g 2 H2O g 16 H2S g 8 SO2 g --- 3 S8 g 16 H2O g Hydrogen sulfide has been used for well over a century as a method of qualitative analysis of metal ions. The next hydride would be H 2 O WATER. Water is more polar than hydrogen sulfide because the oxygen atom is more electronegative than the sulfur atom.
48 g of sulfur and 54 g of aluminum react based on the chemical equation below. Why does hydrogen sulfide have a low boiling point. Lets assume there are HCL aq and H2S aq.
Do notconductelectricity - this is because they do. Originally I thought h2se would have a lower boiling point since it has more shielding and a larger atomicis radius meaning that the attraction between the nucleus and the outer electrons is weaker and there being easier to break. I also thought that the atomic number amount of protons electrons was overruled by shielding so it wouldnt matter if h2se has more.
Wikipedia gives the boiling points of ce H_2S and ce HCl as ce -60 circ C and ce -8505 circC respectively. Answer 1 of 4. 藍 Sulfur is not nearly as electronegative as oxygen so that hydrogen sulfide is not nearly as polar as water.
The report reflects how you feel Chemistry is seen in both chapters and essentially what you learned about Chemistry from reading both chapters so writing in first person form is allowed. Shipped as a liquid confined under its own vapor pressure. Jul 24 2013.
When rationalising boiling point differences the first consideration is always the strength of the intermolecular forces between the molecules in the liquid. It doesnt have hydrogen bonding which results in less intermolecular attractions and therefore a lower boiling point. Explaining the boiling points.
HCL is a strong acid. Because it has very weak intermolecular forces. Sulfur is not nearly as electronegative as oxygen so that hydrogen sulfide is not nearly as polar as water.
Also hydrogen sulfide has weaker intermolecular forces dipole forces vander waals forces compared to water resulting in. Density liquid 83 lb gal. Sulfur is not nearly as electronegative as oxygen so that hydrogen sulfide is not nearly as polar as water.
Only a small of amount of energy is required to break the intermolecular forces so the boling point is low. Herein why is the boiling point of hydrogen sulfide low GCSE. Because of this comparatively weak intermolecular forces exist for H2S and the melting and boiling points are much lower than they are in water.
Very weak London dispersion forces since they require lar. On the other hand while the degree of hydrogen-bonding in H 2T e is less than in H 2S and MUCH less than in H 2O hydrogen telluride is a many electron molecule the which should have greater opportunity for dispersion forces. Why is the boiling point of hydrogen sulfide low.
For an exam answer you would need to state that sulfur dioxide is a small molecule 1 mark so it has weak intermolecular forces between molecules 1 mark therefore only a small amount of energy is required to seperate the molecules of sulfur dioxide 1 mark so it has a. Contact with the unconfined liquid can cause frostbite by evaporative cooling. 2 SO.
Because of this comparatively weak intermolecular forces exist for H2S and the melting and boiling points are much lower than they are in water. Also why hydrogen sulfide has low boiling point. In addition the more creative a person is with this assignment the better.
Ok so HCL aq will mostly disassociate to H and CL- There are 2 ions here for HCL aq higher boiling point elevation. Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs.

Tubesheet Machining A Well Optimized Solution For All Sizes Of Businesses Heat Exchanger Process Engineering Mechanical Design

Why Does H2o Have A Boiling Point Higher Than H2s Quora

Why Does H2o Have The Highest And H2s Has The Lowest Value Of Melting And Boiling Points Quora
0 Comments